The Atom is a fictional superhero appearing in American comic books published by DC Comics. The character was created by editor and co-plotter Julius Schwartz, writer Gardner Fox and penciler Gil Kane. The Atom was one of the first superheroes of the Silver Age of Comic Books and debuted in Showcase #34. Atom Cover art by Steve Dillon for Atom Special #1 Publication information PublisherDC Comics. The atom is considered the basic building block of matter. Anything that has a mass—in other words, anything that occupies space—is composed of atoms. While its name originally referred to a particle that couldn't be divided any more—the smallest thing possible—we now know that each atom is generally made up of smaller particles.
Copper remains one of the single most ubiquitous metals in everyday life. As a conductor of heat and electricity, it is utilized in wires, roofing and plumbing, as well as a catalyst for petrochemical plants, solar and electrical conductors and for a wide range of energy related applications. Subsequently, any method to harvest more of the valuable commodity proves a useful endeavor.
Debora Rodrigues, Ezekiel Cullen Professor of Engineering at the University of Houston Cullen College of Engineering, in collaboration with Francisco C. Robles Hernandez, professor at the UH College of Technology and Ellen Aquino Perpetuo, professor at the University of Sao Paulo, Brazil offered conclusive research for understanding how bacteria found in copper mines convert toxic copper ions to stable single-atom copper.
In their co-authored paper, “Copper Mining Bacteria: Converting toxic copper ions into a stable single atom copper,” their research demonstrates how copper-resistant bacterium from a copper mine in Brazil convert CuSO4 (copper sulfate) ions into zero-valent Cu (metallic copper).
“The idea of having bacteria in mines is not new, but the unanswered question was: what are they doing in the mines?” Robles said. “By putting the bacteria inside an electronic microscope, we were able to figure out the physics and analyze it. We found out the bacteria were isolating single atom copper. In terms of chemistry, this is extremely difficult to derive. Typically, harsh chemicals are used in order to produce single atoms of any element. This bacterium is creating it naturally that is very impressive.”
As useful as copper is, the process of mining the metal often leads to toxic exposures and challenges on drawing out substantial volume for commercial use. Approximately one billion tons of copper are estimated in global reserves, according to the Copper Development Association Inc., with roughly 12.5 million metric tons per year mined. This aggregates to roughly 65 years of remaining reserves. Part of the supply challenge comes from limited available copper in high concentration in the earth’s crust, but the other challenge is the exposure to sulfur dioxide and nitrogen dioxide in the copper smelting and production process to concentrate the metal into useful quantities.
“The novelty of this discovery is that microbes in the environment can easily transform copper sulfate into zero valent single atom copper. This is a breakthrough because the current synthetic process of single atom zerovalent copper is typically not clean, it is labor intensive and expensive,” Rodrigues said.
“The microbes utilize a unique biological pathway with an array of proteins that can extract copper (II) (Cu2+) and convert it into single-atom zero-valent copper (Cu0). The aim of the microbes is to create a less toxic environment for themselves by converting the ionic copper into single-atom copper, but at the same time they make something that is beneficial for us too.”
With a focus in electronic microscopy, Robles examined samples from Rodrigues’ findings in Brazilian copper mines and he determined the single atom nature of the copper. Rodrigues and Aquino’s groups further identified the bacterial process for converting copper sulfate to elemental copper – a rare find.
Research results demonstrate this new conversion process as an alternative to produce single atoms of metallic coper is safer, and more efficient versus current methods (i.e. chemical vapor deposition, sputtering and femtosecond laser ablation).
“We have only worked with one bacterium, but that may not be the only one out there that performs a similar function,” Rodrigues concluded. “The next step for this particular research is harvesting the copper from these cells and using it for practical applications.”Understanding the Atom
The Structure Of An Atom
The nucleus of an atom is surround by electrons that occupy shells, or orbitals of varying energy levels.
The ground state of an electron, the energy level it normallyoccupies, is the state of lowest energy for that electron.
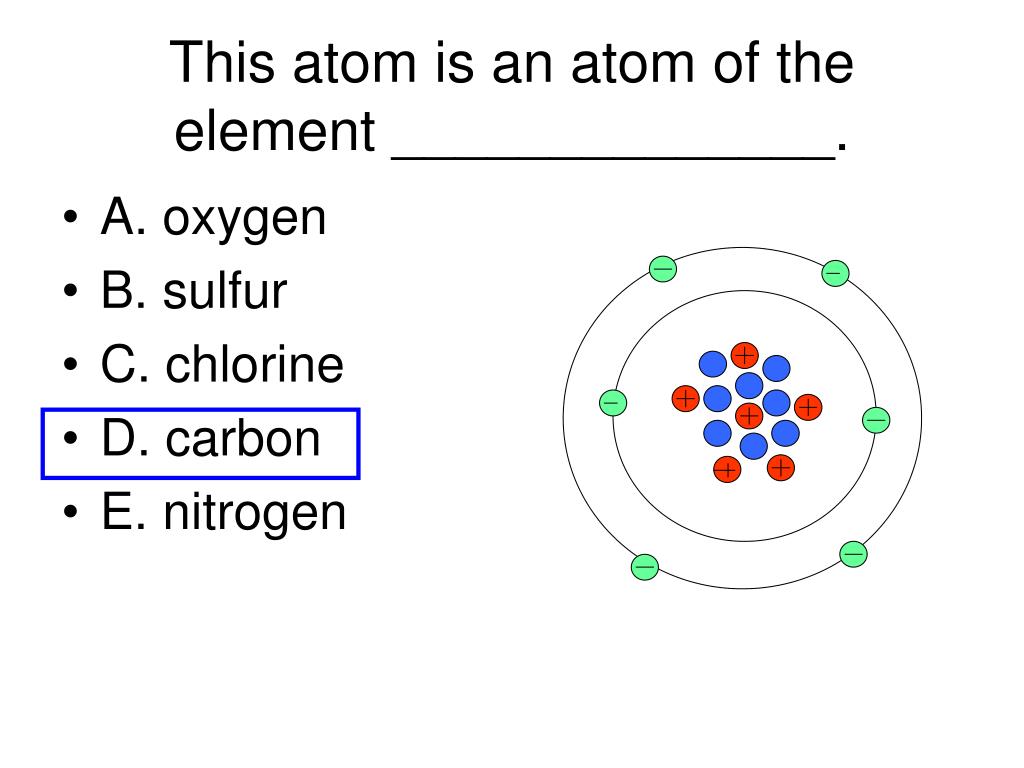
There is also a maximum energy that each electron canhave and still be part of its atom. Beyond that energy, the electronis no longer bound to the nucleus of the atom and it is considered tobe ionized.



When an electron temporarily occupies an energy state greater thanits ground state, it is in an excited state. An electron canbecome excited if it is given extra energy, such as if it absorbs a photon, or packet, of light, or collides with a nearby atom or particle.
Each orbital has a specific energy associated with it. For anelectron to be boosted to an orbital with a higher energy, it mustovercome the difference in energy between the orbital it is in, and theorbital to which is is going. This means that it must absorb a photonthat contains precisely that amount of energy, or take exactly thatamount of energy from another particle in a collision.
Atom Is Gold
Electrons do not stay in excited states for very long - they soonreturn to their ground states, emitting a photon with the same energy asthe one that was absorbed.
Atom Is The Smallest Unit Of
Transitions among the various orbitals are unique for each elementbecause the energy levels are uniquely determined by the protons andneutrons in the nucleus. When the electrons of a certain atom returnto lower orbitals from excited states, the photons they emit haveenergies that are characteristic of that kind of atom. This gives eachelement a unique fingerprint, making it possible to identify theelements present in a container of gas, or even a star.
Updated: November 2013
